
Renzapride (ATL-1251)
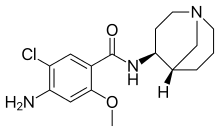
Renzapride is best suited as a treatment of Diabetic Gastroparesis, a condition with a significant unmet treatment options. In the numerous trials that have been done using Renzapride more than 5000 patients have been treated with Renzapride. An extensive cardiac safety evaluation under the FDA mandated Thorough QT trial has shown that Renzapride does not have any cardiac safety issues.
>> Read More
Our Company


EndoLogic is a newcomer in the pharmaceutical and medical device marketplace, but the idea of its creation was conceived a decade ago by its founders, Dr. Zamir S. Brelvi and Dr. Kamal Dutta. Dr. Dutta, a pelvic surgeon, brings more than 30 years clinical knowledge and business development. Dr. Brelvi received his PhD and MD from University of Medicine and Dentistry of New Jersey.

