Welcome to EndoLogic
The mission of EndoLogic LLC is to design, develop, and market new patented technologies in the pharmaceutical and medical devices areas. EndoLogic has two divisions; the Pharmaceutical Division and the Medical Devices Division. Our Pharmaceutical Division focuses on developing small molecules, biologic agents to fulfil current unmet needs to improve medical treatment.RENZAPRIDE (ATL-1251)
The EndoLogic Pharmaceutical Division has recently acquired worldwide rights to Renzapride, ATL 1251 which is a unique small molecule that works to increase the motility of the gastrointestinal tract. Medical conditions that are due to altered gastrointestinal motility are very common, these include, Gastroparesis, Functional Dyspepsia, Gastro-Esophageal Reflux Disease(GERD) and Irritable Bowel Syndrome(IBS) affecting about a 100 million patients in the US. Renzapride will be a suitable treatment option for these conditions.EndoLogic Medical Devices
The EndoLogic Medical Devices Division develops technology that will fill a current need in medical/surgical procedures by improving upon an existing technology or device, or by designing a device to serve a need that is clearly defined and acknowledged by medical professionals. Each product shall be priced to appeal to a managed-care market that stresses lowest cost of total treatment parameters.Our first innovation is the Versa Snare and Retrieval System which has a "US Patent Pending" status. The Versa System is the first ever detachable snare and retrieval system to be developed for endoscopic procedures. The Versa system offers the versatility to use a single device for the removal and retrieval of a polyp or foreign bodies from the gastrointestinal tract.Renzapride (ATL-1251)
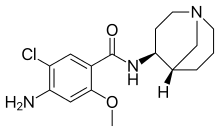
Renzapride is best suited as a treatment of Diabetic Gastroparesis, a condition with a significant unmet treatment options. In the numerous trials that have been done using Renzapride more than 5000 patients have been treated with Renzapride. An extensive cardiac safety evaluation under the FDA mandated Thorough QT trial has shown that Renzapride does not have any cardiac safety issues.
>> Read More
Our Company


EndoLogic is a newcomer in the pharmaceutical and medical device marketplace, but the idea of its creation was conceived a decade ago by its founders, Dr. Zamir S. Brelvi and Dr. Kamal Dutta. Dr. Dutta, a pelvic surgeon, brings more than 30 years clinical knowledge and business development. Dr. Brelvi received his PhD and MD from University of Medicine and Dentistry of New Jersey.

