
Renzapride
Renzapride: History
Renzapride was originally developed in the UK by Smith Kline Beecham Labs in the late 1980s then acquired by Alizyme, another UK based pharmaceutical company. Alizyme chose to develop Renzapride with extensive preclinical, Phase 1, 2 and 3 trials for the indication of the treatment of Irritable Bowel Syndrome with constipation. Although the Phase 3 program demonstrated efficacy in IBS-C, Alizyme decided not to submit the drug for approval and the company was liquidated..EndoLogic has acquired worldwide patent rights for Renzapride. Extensive discussions with FDA consultants and International thought leaders of gastroparesis recommended to develop Renzapride for the treatment of Diabetic Gastroparesis due to a significant unmet need of treatment for this condition.
Renzapride: Mechanism of Action
Renzapride is an orally bioavailable, small molecule. Renzapride was designed to have a unique dual mode of action. Renzapride is potent 5-HT4 agonist and 5-HT3 antagonist. Stimulating 5-HT4 receptors in the gut wall promotes the release of acetylcholine, which works to increase peristalsis (movement)of the gastrointestinal tract.The 5-HT3 antagonist effect allows Renzapride to also function as an anti-emetic.
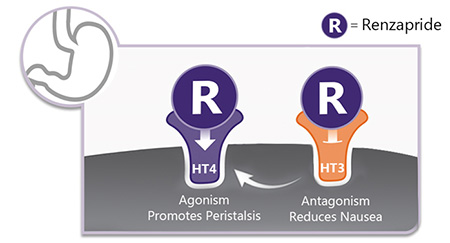
These unique dual mode of actions of Renzapride allow it to be most suitable to be used in the treatment of Diabetic Gastroparesis, a condition with a significant unmet treatment options.
Renzapride: Development Proposal
In the numerous trials that have been done using Renzapride more than 5000 patients have been treated with Renzapride. An extensive cardiac safety evaluation under the FDA mandated Thorough QT trial has shown that Renzapride does not have any cardiac safety issues.EndoLogic proposes to develop Renzapride for the treatment of Diabetic Gastroparesis by conducting a Phase 2 dosing trial and subsequently two Phase 3 trials leading to FDA approval.
Renzapride (ATL-1251)
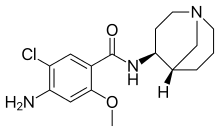
Renzapride is best suited as a treatment of Diabetic Gastroparesis, a condition with a significant unmet treatment options. In the numerous trials that have been done using Renzapride more than 5000 patients have been treated with Renzapride. An extensive cardiac safety evaluation under the FDA mandated Thorough QT trial has shown that Renzapride does not have any cardiac safety issues.
>> Read More
Our Company


EndoLogic is a newcomer in the pharmaceutical and medical device marketplace, but the idea of its creation was conceived a decade ago by its founders, Dr. Zamir S. Brelvi and Dr. Kamal Dutta. Dr. Dutta, a pelvic surgeon, brings more than 30 years clinical knowledge and business development. Dr. Brelvi received his PhD and MD from University of Medicine and Dentistry of New Jersey.

