
Media
Atlantic Healthcare plc Acquires Global Rights to Renzapride for the Treatment of Gastrointestinal Motility Disorders
Atlantic Healthcare plc acquires global rights to renzapride for the treatment of gastrointestinal motility disorders.
CAMBRIDGE, ENGLAND (PRWEB) JUNE 26, 2019Atlantic Healthcare plc (“Atlantic Healthcare” or “Company”), a specialist pharmaceutical company focused on developing and commercializing therapeutics that address unmet patient needs and rare diseases, today announced that the Company has entered into an agreement to acquire the global rights to renzapride from EndoLogic LLC (“EndoLogic”). Renzapride is a clinical stage product, which we believe has wide applicability for the treatment of motility disorders of the gastrointestinal (GI) tract, many of which represent significant unmet medical needs.
Under the terms of the agreement, EndoLogic will receive a signature payment to the value of $3m, enabling the company to acquire an equity stake in Atlantic Healthcare plc. EndoLogic is also eligible to receive up to $48m in milestone payments for the development of gastrointestinal indications, a number of which are expected to be orphan designations. In addition, the agreement includes a commercial milestone payment and single digit royalties on product sales.
Toby Wilson Waterworth, Chief Executive Officer, Atlantic Healthcare said: “Renzapride is an important addition to Atlantic Healthcare’s portfolio, which complements the Company’s existing pipeline. The molecule’s attractive safety profile and unique dual mode of action will help differentiate it from other products targeting gut motility. We look forward to building a robust clinical pipeline with renzapride, to address unmet patient needs in this GI clinical area.”
Zamir S Brelvi, M.D., Ph.D., Co-Founder and Chief Executive Officer, EndoLogic said:
“Dr Dutta, my co-founder, and I are delighted to have agreed terms with Atlantic Healthcare, to develop further renzapride’s potential. As leaders of EndoLogic, which seeks innovative solutions for unmet patient needs, we are confident that Atlantic Healthcare’s expertise in orphan drug and gastrointestinal indications will be invaluable. We are pleased to become a shareholder and look forward to contributing our medical expertise in the years ahead.”
About Renzapride
Renzapride is an orally bioavailable small molecule with a dual mode of action. It is a full agonist at 5-HT4 receptors and stimulates gut motility. It is also a partial antagonist at 5-HT3 receptors, which has the effect of reducing nausea and vomiting, symptoms experienced by many patients with impaired gut motility. This dual mode of action means renzapride has the potential to treat a variety of gastrointestinal disorders.
In clinical trials involving over 1,000 patients, renzapride has demonstrated efficacy in the upper and lower gastrointestinal tract. These trials have shown that it can enhance gastric emptying, reduce small bowel transit time and increase colonic motility.
In development, renzapride has been found to be safe and well tolerated. It does not demonstrate the cardiac toxicity and prolonged QT interval (the time it takes for the ventricles of the heart to contract and relax) that is exhibited by some other products with the same pharmacology.
About Atlantic Healthcare
Atlantic Healthcare plc is focused on developing and commercializing therapeutics that can address unmet needs of patients who are managed by healthcare professionals in hospital and specialist care environments. The Company owns the exclusive worldwide rights to alicaforsen, a promising drug that is in development for the treatment of inflammatory bowel disease (IBD) and other gastrointestinal indications.
We intend to commercialize our products in Europe and the U.S. using a specialist sales team targeting gastroenterologists in hospitals and specialist care centers. We plan to partner with established pharmaceutical companies to commercialize products in the rest of the world.
Atlantic Healthcare is led by an experienced international Board and Leadership Team, with deep roots and a proven track record in the pharmaceutical industry.
About EndoLogic
EndoLogic LLC is a U.S. company that designs and develops new patented technologies in the pharmaceutical and medical devices sector. The Company’s founders, Dr. Zamir S. Brelvi and Dr. Kamal Dutta are highly experienced U.S. clinicians.
Dr. Zamir S Brelvi, M.D., Ph.D., is a US trained gastroenterologist, inventor and academic researcher with a vast experience in endoscopic procedures and device development. Dr. Kamal Dutta, MD, FACOG, FACS, is a pelvic surgeon, with more than 30 years of experience in designing, developing and patenting technologies in the pharmaceutical and medical device sectors.
Forward Looking Statements
This press release contains forward looking statements that are based on management’s beliefs and assumptions as of the date of this press release. Such statements are subject to numerous important factors, risks and uncertainties that may cause actual events or results to differ materially from those indicated by such statements. These forward-looking statements involve risks and uncertainties, including, among others, that the development of any products for their stated uses may not proceed due to safety, efficacy or other reasons. In addition, risks and results in clinical trials may not be indicative of risks or results from later stage or larger scale trials, and there is no assurance of regulatory approval. Existing and prospective investors should not place undue reliance on the forward-looking statements contained in this press release and instead should make their own determinations as to the reliability of such statements. Atlantic Healthcare undertakes no intent or obligation to update the information contained in this press release.
For more information, please contact:
Adam Michael (Head of Communications) Media Contact
+44 1799 512 055
+44 777 588 1813
adam.michael(at)atlantichc(dot)com
U.S. Investor Relations and Media
Lazar Partners
David Carey / Amy Feldman
+1 212-867-1762
European Investor Relations and Media
Consilium Strategic Communications
Mary-Jane Elliott / Matthew Neal
+44 20 3709 5700
atlantichealthcare(at)consilium-comms(dot)com
EndoLogic Announces FDA Accepts Cardiac Safety Trial for Renzapride in Patients with Gastroparesis
Study demonstrates no evidence of QTc prolongation.EndoLogic remains on plan to initiate 12-week renzapride Phase 2 trial pending collaboration with a development partner
CLIFTON, N.J., May 15, 2018 (GLOBE NEWSWIRE) -- EndoLogic announced that the FDA accepted the cardiac safety trial for renzapride in patients with gastroparesis. That study demonstrated no evidence of QTc prolongation in the EKG findings. The U.S. Food and Drug Administration (FDA) views this study as sufficient to characterize renzapride’s QTc prolongation potential at both therapeutic (4 mg per day) and supra-therapeutic (20 mg per day) doses of the compound in patients with gastroparesis.
“Renzapride is a member of the same 5-HT4 agonist class as the once highly efficacious and commercially-successful cisapride (Propulsid®; more than $1 billion in annual sales),” said Kamal Dutta, M.D. co-founder and president of EndoLogic. “However, renzapride is without the serious cardiac side effects, including deaths that emerged with cisapride. In addition, renzapride is an antiemetic due to its strong 5-HT3 antagonism. There is a need for a safe and well-tolerated treatment for gastroparesis. We are confident that renzapride could fill that void by demonstrating efficacy in treating patients’ symptoms such as severe abdominal pain, nausea, vomiting, bloating and early satiety.”
Renzapride, a 5-HT4 agonist and 5-HT3 antagonist, has been studied in more than 5,000 patients and was also well tolerated and showed no evidence of cardiotoxicity. A pilot Phase 2 study in patients with diabetic gastroparesis showed significant improvement in gastric emptying in a dose-dependent manner. In vivo studies have demonstrated that renzapride, with its 5-HT4 agonist activity has similar prokinetic activity as cisapride and, with its 5-HT3 antagonist activity, has eight times more antiemetic activity than cisapride. The company plans to conduct the Phase 2 study to identify the best dose to treat gastroparesis for the second pivotal trial.
“The FDA acceptance of our cardiac safety study confirms our own view that renzapride exhibits no cardiotoxicity and is thus a game changer for this drug,” said Zamir S. Brelvi M.D., Ph.D., co-founder and chief executive officer of EndoLogic. “Renzapride’s cardiac safety and its dual action as a prokinetic and antiemetic with virtually no drug-drug interactions positions renzapride as 'best in class" and a long-awaited treatment for gastroparesis. "We are now fully ready to collaborate with a partner to move forward with our upcoming Phase 2 trial.”
Gastroparesis is a common condition affecting more than 20 million people in the U.S. including five million diabetics. Currently, treatment options are limited with only one drug, metoclopramide (brand name Reglan®), a four times daily oral dopamine D2 receptor antagonist, approved for the treatment of gastroparesis; however, treatment with metoclopramide for more than 12 weeks should be avoided due to the risk of tardive dyskinesia. Renzapride, a twice-daily oral medication, could be a promising and safe option for patients with gastroparesis. Being a prokinetic agent, it also has potential benefit in other indications such as proton pump inhibitor-refractory gastroesophageal reflux disease (GERD) and functional dyspepsia. Both of these conditions combined affect 50 million patients in the U.S.
EndoLogic Acquires Renzapride from Alizyme, plc
Clinical-stage asset to be developed for gastroparesis, a poorly-met medical need.
CLIFTON, N.J., Jan. 03, 2017 (GLOBE NEWSWIRE) -- EndoLogic, a company focused on the development and commercialization of products that treat gastrointestinal diseases, has acquired the worldwide rights to the clinical-stage asset, renzapride, from Alizyme, plc. Renzapride has been in clinical development for use as a prokinetic agent for the gastrointestinal tract. EndoLogic plans on developing the compound for the treatment of gastroparesis.
Renzapride, a 5-HT4 agonist and 5HT-3 antagonist, has been studied in more than 5,000 patients including one Phase 3 trial for the treatment of constipation-dominant irritable bowel syndrome (IBS-C). Renzapride demonstrated a small but statistically significant benefit in the Phase 3 study in IBS-C, however, Alizyme decided to not continue to pursue development of the drug for this indication. The drug was well tolerated and showed no evidence of cardiotoxicity. A pilot Phase 2 study in patients with diabetic gastroparesis showed that doses of 0.5 mg, 1.0 mg and 2.0 mg, once-daily, showed significant improvement in gastric emptying in a dose-dependent manner. The company plans to conduct a Phase 2 study to identify the best dose to treat diabetic gastroparesis for Phase 3 testing.
Gastroparesis is a common condition affecting more than 20 million people in the U.S. including 5 million diabetics. Currently, only one drug, metoclopramide (brand name Reglan®) a dopamine D2 receptor antagonist, is approved for the treatment of gastroparesis. Unfortunately, patients are at risk for serious side effects some of which are permanent, such as tardive dyskinesia, hence limiting its use to no more than 12 weeks. It is also relatively inconvenient to administer, taken orally four times daily usually 30 minutes prior to a meal and at bedtime. Renzapride, a twice-daily oral medication, could be both a more convenient and safer alternative if successfully developed and approved by regulatory authorities. It also has potential benefit in other indications in which a prokinetic agent could be beneficial such as gastroesophageal reflux disease (GERD).
“We purchased renzapride from its original developer because we saw significant opportunity as a therapeutic to address the poorly-served therapeutic area of diabetic gastroparesis,” said Zamir S. Brelvi M.D., Ph.D., co-founder and chief executive officer of EndoLogic. “Although the compound demonstrated very promising activity in a Phase 2 pilot study in diabetic gastroparesis, the prior owner of the asset decided to develop the drug for what they viewed as a more significant market opportunity in IBS-C, another indication in which prokinetic compounds could be of value. Its promising activity in early studies in gastroparesis, its significant clinical experience, particularly its safety profile, and its potential in indications beyond gastroparesis suggest a relatively low-risk and high-reward asset. We are now looking for investors who are interested in working with the company to assist in developing renzapride and successfully launching the product.”
Drs. Brelvi and Kamal Dutta, Co-founder and President of EndoLogic, will be in San Francisco from Jan 9 to 13, 2017 to meet with members of the investment community. Please contact Robert Flamm from The Ruth Group if you wish to schedule a meeting.
About EndoLogic
The mission of EndoLogic LLC is to design, develop, and market new products to treat gastrointestinal diseases. The company has developed a series of medical device products for the removal of colon polyps and foreign bodies. 510K applications have been submitted to the U.S. Food and Drug Administration for review. The company has acquired renzapride, a 5HT-4 agonist and 5HT3 antagonist, to develop it for the treatment of gastroparesis, a poorly-met medical need and for other gastrointestinal indications. The company’s founders, Dr. Zamir S. Brelvi a U.S. trained gastroenterologist and academic researcher with a vast experience in endoscopic procedures and device development, and Dr. Kamal Dutta. Dr. Dutta, a pelvic surgeon, brings more than 30 years clinical knowledge and business development to the company.
Corporate contact:
Zamir S. Brelvi M.D., Ph.D.
Chief Executive Officer
zbrelvi@endologicusa.com
Investor contact:
Robert Flamm, Ph.D.
Senior vice president
The Ruth Group
P: 646-536-7017
rflamm@theruthgroup.com
Renzapride (ATL-1251)
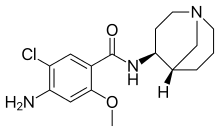
Renzapride is best suited as a treatment of Diabetic Gastroparesis, a condition with a significant unmet treatment options. In the numerous trials that have been done using Renzapride more than 5000 patients have been treated with Renzapride. An extensive cardiac safety evaluation under the FDA mandated Thorough QT trial has shown that Renzapride does not have any cardiac safety issues.
>> Read More
Our Company


EndoLogic is a newcomer in the pharmaceutical and medical device marketplace, but the idea of its creation was conceived a decade ago by its founders, Dr. Zamir S. Brelvi and Dr. Kamal Dutta. Dr. Dutta, a pelvic surgeon, brings more than 30 years clinical knowledge and business development. Dr. Brelvi received his PhD and MD from University of Medicine and Dentistry of New Jersey.

